Antigen availability drives differentiation of B cells after immunisation
After the body is exposed to an antigen, activated B cells begin to differentiate along three distinct lineages that contribute to the immune response. How and when the fate of each cell is decided has remained unclear so far. An international team of immunologists tackled these questions by using single-cell RNA sequencing to map the trajectory of individual cells as they differentiated. They found that antigen availability orchestrates the differentiation of activated B cells: plasmablasts are produced first and, as access to the antigen drops, germinal centre B cells and early memory B cells emerge. Their findings are now reported in the journal Immunity.
Our immune system detects foreign particles, called antigens, using receptors on the surface of immune cells. When antigens come from pathogens, B cells differentiate into plasmablasts, which secrete enormous amounts of antibodies – large proteins that help fight the invading pathogen. Furthermore, in a process called germinal centre reaction, B cells improve the affinity of their receptors and finally differentiate into plasma cells, which now secrete antibodies with the same high affinity for the enemy to eliminate. These high affinity antibodies are crucial to clear infections.
The immune system is capable of ‘remembering’ past encounters with pathogens, so that it can respond more quickly to a recurring infection. Plasma cells are long-lived and provide protection for several weeks up to months. In addition, the memory B cells that are generated at first can reside in the human body for our entire lives, and can be reactivated to give rise to antibody-secreting plasmablasts. This process is much faster than the initial response, which makes the fight against a known pathogen more effective.
Traditional vaccines make use of that mechanism and allow the immune system to build its memory with a ‘mock exam’, a harmless version of an antigen, before real infection takes place.
The underlying molecular mechanisms and the timing of the trajectories of activated B cells are still unclear. In an international effort, scientists at the IMP, Karolinska Institutet, the Medical University of Vienna, and their collaborators have followed the fate of individual immune cells using single-cell RNA sequencing. They recently reported their findings in the journal Immunity.
Plasmablasts produced first
“Once activated B cells start proliferating, we found that they first produce plasmablasts in a temporary wave of division, whereas germinal centre B cells are generated one day later,” says René Rauschmeier, PhD student in the Vienna BioCenter PhD Program and co-first author.
The researchers observed that, after the swell of plasmablast production had passed, a large proportion of activated B cells differentiated into early memory B cells. These cells make a substantial contribution to the long-lived memory B cell pool and could therefore be recruited again for plasmablast production, should the need occur.
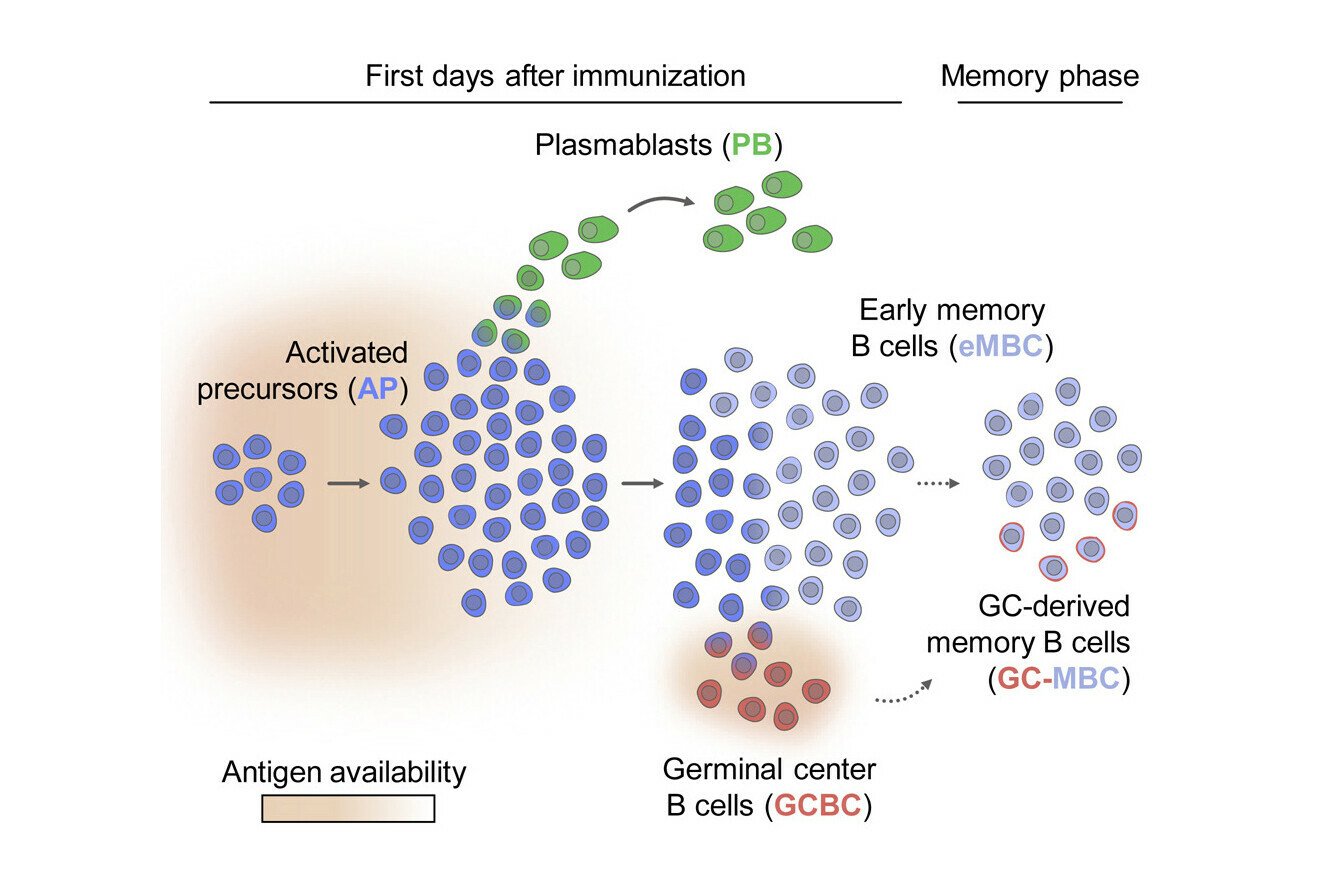
Antigen availability orchestrates the fate of activated B cells
The study shows that the fate of activated B cells responds to the amount of antigen that is available at a given time. When the scientists experimentally increased the availability of antigen for activated B cells, they found that it prolonged the production of plasmablasts and delayed the generation of early memory B cells, which only happened when antigen availability declined. If more of the antigen was provided, these early memory B cells could be quickly reactivated to give rise to another wave of plasmablasts.
“In a scenario where the body fails to contain an infection, antigens would abound and more plasmablasts would need to be produced,” explains Meinrad Busslinger, Deputy Scientific Director at the IMP. “Producing plasmablasts costs a high amount of energy – a demand-based system that responds to the availability of antigen avoids wasting that energy.”
The researchers provide insights into the early stages of the immune response following immunisation. Their findings may help improve future vaccination strategies.
Original publication
*These authors contributed equally to the study.
Further reading