Disrupting ribosome production – renewed potential for cancer therapy
Proliferating cells are in constant need of ribosomes, the molecular machines that help them produce proteins. Tumour cells, for instance, divide quickly and strongly rely on ribosome production to maintain their growth. A team of scientists has visualised a novel mechanism that inhibits the production of ribosomes in yeast. Their results, now published in the journal Nature Communications, could be used to develop novel classes of inhibitors to target cancer cells.
Proteins are the essence of life: they give structure to cells and organelles, catalyse metabolic reactions, build, break, tug, and pull – they carry out most of the actions that sustain a cell’s existence.
A cell can produce tens of thousands of different proteins with distinct roles with the help of ribosomes – large molecular ‘pastry chefs’ that put together simple ingredients (amino acids) into a variety of complex products (proteins) based on strict recipes (RNA). The career of a ribosome is limited in time, so when cells proliferate and need large quantities of proteins, they heavily rely on the production of new ribosomes to sustain their divisions. Cancer cells, for instance, need a constant supply of ribosomes for their explosive proliferation.
Building a ribosome requires a complex pathway that involves a diverse array of molecules, including a family of proteins called AAA-proteins, for ATPases Associated with diverse cellular Activities. AAA-proteins are crucial for many cellular processes in all living organisms. Due to their pivotal roles, AAA-proteins emerged as drug targets in recent years.
In the baker’s yeast (Saccharomyces cerevisiae), the AAA-protein Drg1 (diazaborine resistant gene 1) is essential for the constant supply of ribosomes. Diazaborine, a small chemical, can bind to Drg1 and shut down its activity.
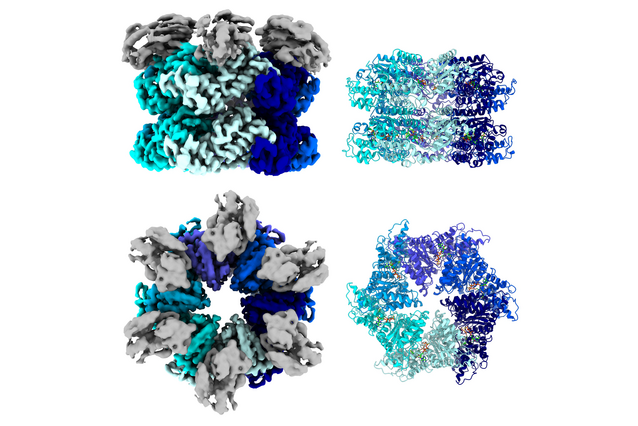
Until today, researchers did not know the molecular details of this interaction. Scientists at the University of Graz and at the IMP determined the microscopic structure of Drg1, thereby unveiling the mechanism through which diazaborine inhibits its activity. Their results, now published in Nature Communications, unlock new opportunities for cancer therapy.
One special bond
The molecules that inhibit the activity of proteins usually fit into a ‘binding pocket’ in their target, where they tie multiple chemical bonds. Diazaborine, however, does it its own way: it barely interacts with Drg1 itself, and instead creates a single bond to another small molecule, a nucleotide cofactor, that is transiently attached to Drg1 (see image below). This efficiently inactivates the protein.

“We didn’t know how diazaborine worked until we could finally visualise the structure of Drg1 with cryo-Electron Microscopy,” says Michael Prattes, postdoc at the University of Graz and first author of the study. “The single, indirect bond between diazaborine and its target is quite unique in the protein world and it seems to be very specific.”
Drg1 and its inhibitor, diazaborine. Credit: University of Graz
New avenues in cancer therapy
“Specificity is the holy grail of drug design: therapies should target diseased cells with as little impact on their healthy neighbours as possible,” says Helmut Bergler, Associate Professor at the University of Graz. “Using this mechanism to target proteins related to human disease could have enormous therapeutic potential.”
Building ribosomes is the most energy-consuming cellular process, but it is essential for cells to multiply. The main characteristic of cancer cells is their fast, erratic proliferation – one way to stop it could thus be to impede the production of ribosomes.
“Cancer cells heavily rely on ribosome production to grow. This pathway is the Achilles’ heel of tumours, and there are ways to target it,” explains David Haselbach, group leader at the IMP. “Our work opens up possibilities for the design of new cancer drugs and treatments.”
Original publication
Prattes, M., Grishkovskaya, I., Hodirnau, V.-V., Rössler, I., Klein, I., Hetzmannseder, C., Zisser, G., Gruber, C., Gruber, K., Haselbach, D., Bergler, H.: “Structural basis for inhibition of the AAA-ATPase Drg1 by diazaborine”. Nature Communications, 9 June 2021. DOI: http://dx.doi.org/10.1038/s41467-021-23854-x.