Embryonic development: the Toddler mystery solved

As an embryo develops, its cells migrate to the appropriate location as they turn into new cell types. During her postdoctoral work, Andrea Pauli had identified the protein ‘Toddler’ as a crucial factor for the early movements of mesodermal cells, yet the underlying mechanism remained unknown. In a recent study published in the journal Science Advances, Pauli and her lab report a new and intriguingly simple mechanism that explains how a single receptor can both generate and sense the concentration gradient of a molecular signal to steer cell migration.
The life of an animal starts when an egg and sperm come together and merge to form a zygote, the very first cell of the embryo. This cell then quickly divides to give rise to a cluster of undifferentiated cells.
As development continues into gastrulation, these cells differentiate into three distinct tissues – the endoderm, mesoderm and ectoderm – and migrate in a cell-type specific manner to eventually give rise to the animal’s body plan. While the mechanisms inducing distinct cell fates are relatively well understood, the way cells navigate the complex and dynamic environment of an embryo to migrate to their destination has remained unclear.
In 2014, Andrea Pauli, then a postdoctoral researcher with Alex Schier at Harvard University, first discovered ‘Toddler’, a small, secreted protein named after the reduced, erratic movements of embryonic cells lacking this protein. In their seminal study, Pauli and colleagues described Toddler’s essential role for mesodermal cell migration. However, they had not pinpointed how it performs its function.
In a new study published in the journal Science Advances, scientists in Pauli’s lab lead by PhD student Jessica Stock, in collaboration with Edouard Hannezo (Institute of Science and Technology Austria), uncover how mesodermal cells self-generate a Toddler concentration gradient that they then follow to direct their migration.
Toddler, an unusual chemokine signal
When cells migrate during development, they often follow a molecular signal – a chemokine – which guides them to their destination. The signal is usually secreted from a localised source, which generates a concentration gradient. As cells expressing the signal receptor detect this signal, they orient themselves towards higher concentrations of the signal – in other words, towards the source.
“Our initial experiments had excluded Toddler as a regular chemokine signal because changing the location of the Toddler source didn’t affect cell migration,” explains Pauli. “What we discovered in our latest research is that the concentration gradient doesn’t result from a localised Toddler-emitting source, but instead from a localised sink formed by the migrating cells themselves. As they migrate, they eat up Toddler and decrease its concentration locally.”
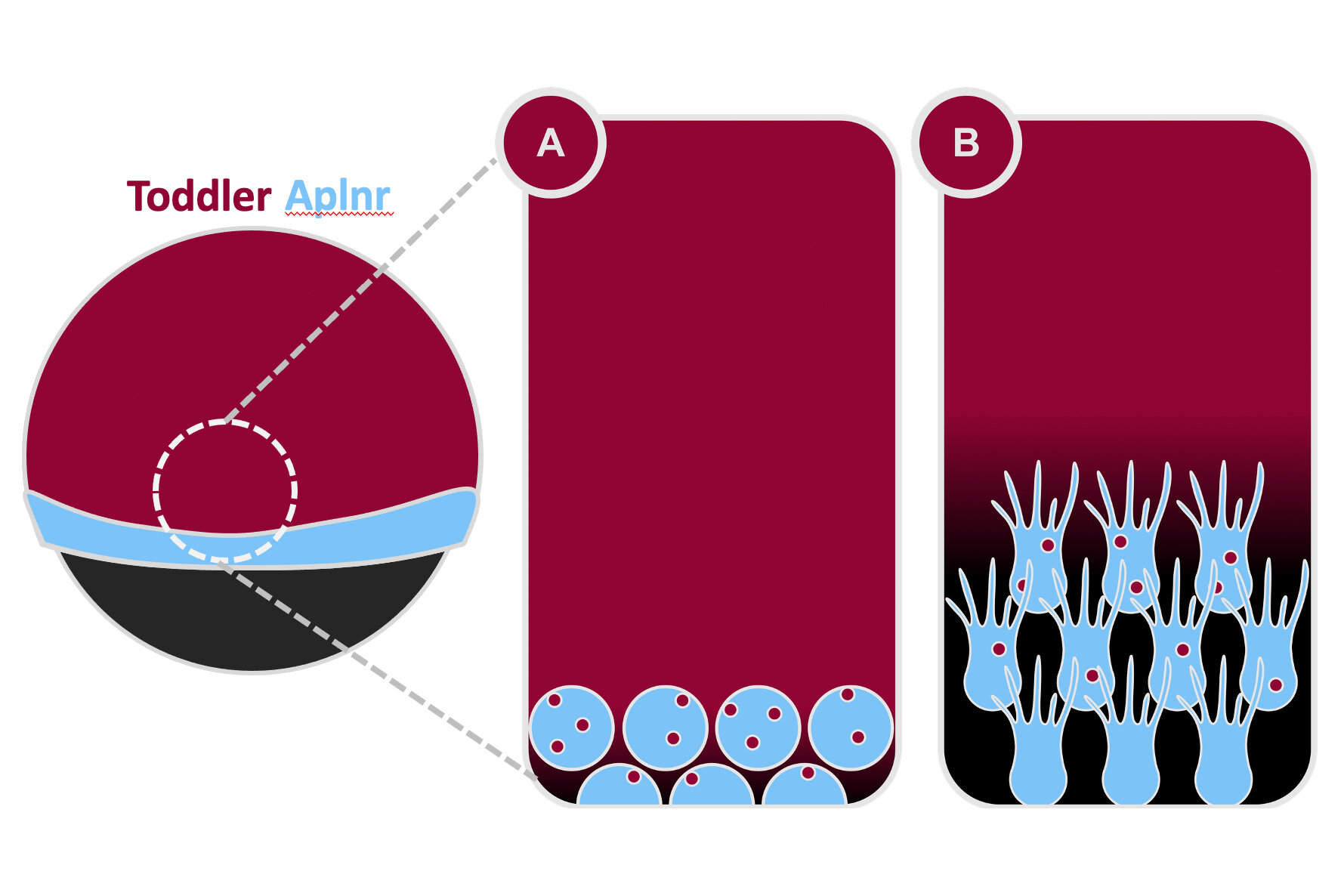
In their new study, the researchers discovered a dual function for Toddler’s receptor, the GPCR Aplnr: not only does it sense the Toddler gradient, but it also ‘scavenges’ for Toddler to internalise it, thereby generating the gradient in the first place. Before gastrulation, mesodermal cells expressing the receptor arise at the boundary between embryo and yolk. Because of this specific location, Toddler will only be ‘eaten up’ locally at this end of the embryo but not at the other end. This creates a Toddler gradient throughout the embryo that directs mesodermal cells to migrate to the future head of the embryo.
“The way Toddler operates has been a long-standing puzzle in my lab. Our collaboration with Edouard Hannezo has been instrumental to answering the enigma – but I am first and foremost very proud of my recently graduated PhD student Jessica Stock for her fantastic work,” says Pauli. “Our findings reveal that a single receptor-based self-generated gradient acts as the guidance mechanism that can robustly steer the directional migration of mesodermal cells through the embryo. It could well be that similar self-generated signalling gradients are a much more wide-spread mechanism underlying other enigmatic directed cell migration events during development and beyond.”
Original publication
Further reading