Fighting tuberculosis with bacterial self-sabotage
With 1.6 million casualties in 2021, tuberculosis remains the world’s deadliest infectious disease arising from a single agent. Resistant strains of Mycobacterium tuberculosis, the bacterium causing the disease, are now common, resulting in an epidemic comeback since 2020, according to WHO. Scientists at the IMP and collaborators have harnessed the BacPROTAC technology to develop an antimicrobial agent that forces targeted bacterial proteins into suicide. Their study, now published in the journal Cell, brings new hope to combat bacterial pathogens.
Tuberculosis and humanity go way back – traces of the disease were found in 3,000-year-old Egyptian mummies. When it is symptomatic, the disease mostly affects the lungs, leading to bloody cough and weight loss; and, in some cases, death.
After millennia of coexistence and despite the development of an arsenal of treatments, tuberculosis remains the deadliest infectious disease caused by a single agent, according to WHO: 1.6 million deaths among 10.6 million cases in 2021, while malaria killed 650,000, and HIV-related illnesses 619,000.
Tuberculosis cases have surged in 2020 and 2021 in the Global South, reversing years of slow decline, possibly due to the rise of antibiotic-resistant bacterial strains. Resistance threatens to cause global outbreaks and begs for the development of drugs with novel modes of action.
Promising antimicrobial compounds
One of the most promising drug targets in M. tuberculosis is the Clp system, a complex of enzymes that unfold and chop up misfolded and unwanted proteins. Were this machinery to run amok, abnormal protein concentrations could occur and cause a lethal imbalance in the bacterial cell. Some naturally occurring chemicals extracted from free-ranging bacteria, such as cyclomarin A and ecumicin, can bind to ClpC1, a crucial component of the Clp system, and impair its activity. However, as for many other antimicrobial compounds, there is one potential issue: bacterial defence mechanisms.
To tackle this problem, scientists in the lab of Tim Clausen at the IMP and international collaborators dissected the effects of cyclomarin A and ecumicin on the protein composition inside bacterial cells. In a study now published in the journal Cell, the scientists report that these compounds mimic misfolded proteins to bind ClpC1 and dysregulate protein concentrations tremendously.
“Cyclomarin A and ecumicin hijack the system and get it to fail: ClpC1 is blocked from binding the right proteins and launches its degrading activity, targeting nearby molecules that should be kept intact,” says David Hoi, co-first author and recent graduate of the Vienna BioCenter PhD Program.
Beating a bacterial defence mechanism
The scientists identified two proteins whose concentration drastically increase after bacterial cells are treated with cyclomarin A or ecumicin: ClpC2 and ClpC3, respectively. Upon further investigation, they discovered that these proteins make up a defence mechanism, protecting ClpC1 by binding the antimicrobial compounds and keeping them away from the core Clp machinery. In other words, they act as bouncers.
“ClpC2 and ClpC3 might come in particularly handy when bacterial cells are in stressful environmental conditions. They prevent ClpC1 from being overloaded with proteins,” explains Sabryna Junker, co-first author and postdoc at the IMP. “They also mitigate the effect of antimicrobial compounds that target ClpC1 by preventing their interaction. This defence mechanism was an unexpected discovery – who knows what other mechanisms we might discover in other bacterial species?”

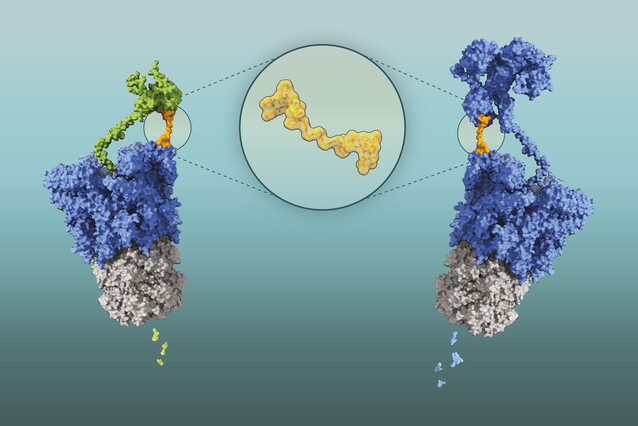
The scientists devised a ruse to circumvent these molecular bodyguards: they created a two-headed compound of cyclomarin A. These molecules were able to turn Clp against itself: each cyclomarin head bound separate ClpC1 molecules, bringing them together and encouraging them to unfold and degrade each other.
This technology is based on BacPROTACs (for “bacterial proteolysis targeting chimeras”), molecules that can be designed to cause the selective degradation of any bacterial protein. BacPROTACs are composed of two linked modules: a target-binding module that selectively attaches to a protein of interest, and a chemical group that tethers the target protein to the Clp enzyme, thereby inducing degradation. In this specific case, both modules are the same molecules, derived from cyclomarin A, hence the updated name ‘Homo-BacPROTAC’.
Homo-BacPROTACs not only degrade ClpC1 effectively, but they also target ClpC2, thereby overcoming the bacterium’s defence mechanism. This inhibits the growth of M. tuberculosis more than a hundred times more effectively than single cyclomarin A molecules. “Our collaborators contributed their expertise in microbiology and chemistry and were key to the success of our study,” says Tim Clausen.
BacPROTACs have a huge potential in the fight against antibiotic resistance: they are specific, effective, and adaptable. They also target a Clp domain that is essential for bacteria to cope with stressful situations and is thus unlikely to mutate.
“All living organisms have protein recycling machineries, and the PROTAC technology could be adapted to target specific groups of species, whether it’s within bacteria, fungi, or plants. One day, they could be used to treat tuberculosis and other infections in humans, but also in animals and crops,” Clausen says. “However, before this technology can be marketed, it will have to be further tested, adapted, and tweaked, so that it’s as effective as possible, and safe for patients.”
Original publication
David M. Hoi*, Sabryna Junker*, Lukas Junk#, Kristin Schwechel, Katharina Fischel, David Podlesainski, Paige M. E. Hawkins, Lasse van Geelen, Farnusch Kaschani, Julia Leodolter, Francesca Ester Morreale, Stefan Kleine, Somraj Guha, Klaus Rumpel, Volker M. Schmiedel, Harald Weinstabl, Anton Meinhart, Richard J. Payne, Markus Kaiser, Markus Hartl, Guido Boehmelt, Uli Kazmaier, Rainer Kalscheuer, Tim Clausen#: “Clp-targeting BacPROTACs impair mycobacterial proteostasis and survival”. Cell (2023), DOI: 10.1016/j.cell.2023.04.009.
*These authors contributed equally.
#Co-corresponding authors
About the IMP at the Vienna BioCenter
The Research Institute of Molecular Pathology (IMP) in Vienna is a basic life science research institute largely sponsored by Boehringer Ingelheim. With over 200 scientists from 40 countries, the IMP is committed to scientific discovery of fundamental molecular and cellular mechanisms underlying complex biological phenomena. The IMP is part of the Vienna BioCenter, one of Europe’s most dynamic life science hubs with 2,650 people from over 80 countries in six research institutions, two universities, and 40 biotech companies. www.imp.ac.at, www.viennabiocenter.org
Further reading